WHO/IUIS Allergen Nomenclature Sub-Committee
Financial contributions from IUIS, EAACI, and AAAAI
Please download the following document in pdf file here .
Glycan Epitopes Currently Recognized as Targets for IgE Antibodies
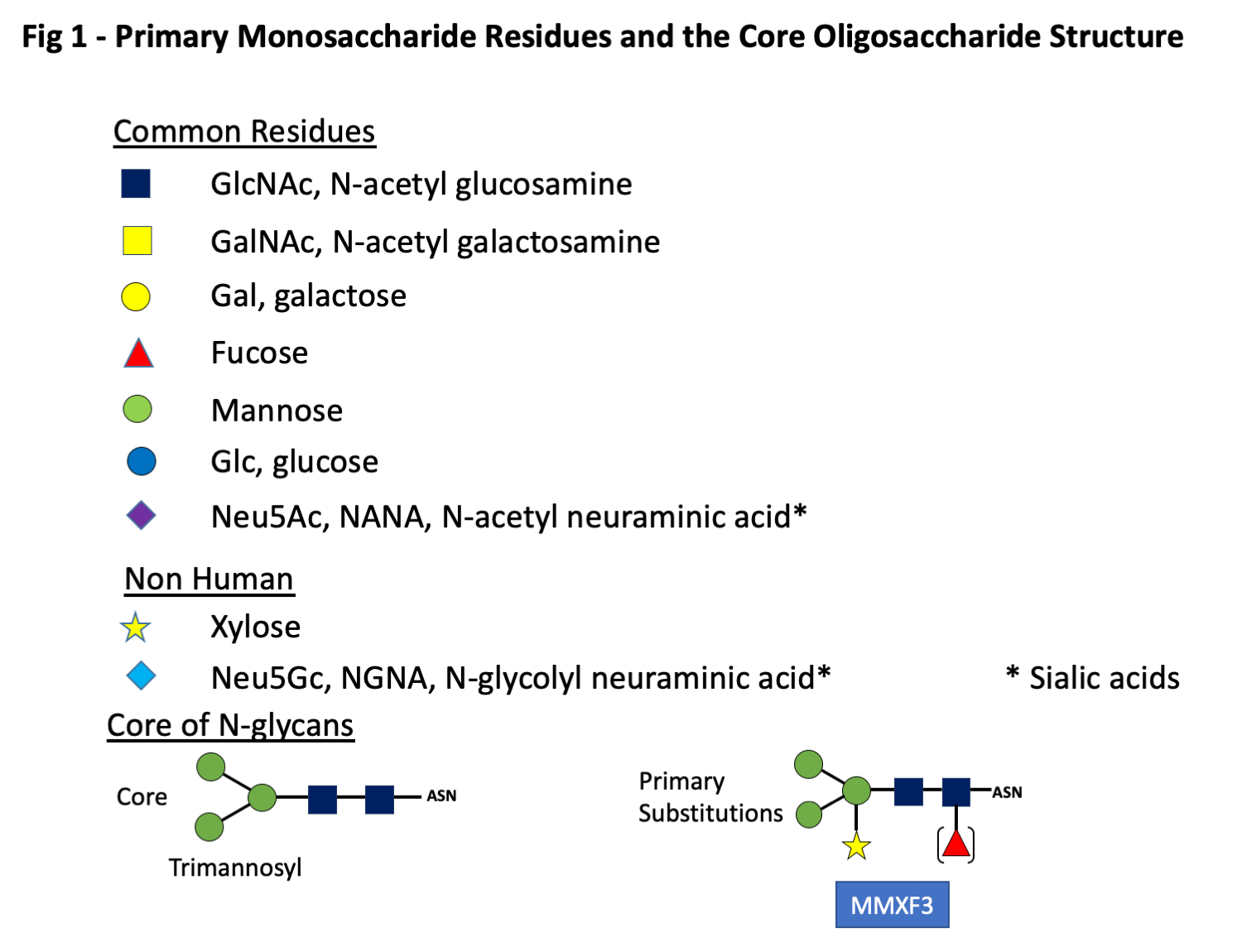
It has long been recognized that sera from some patients have IgE antibodies (ab) specific for oligosaccharide rather than protein epitopes. In the earliest studies related to pollen antigens, these oligosaccharide epitopes were designated as cross-reactive carbohydrate determinants (CCD) because they proved to be responsible for extensive cross-reactions in vitro (1-3). Subsequently, several other groups of antigens were identified where the target of the IgE ab response was found to comprise oligosaccharide epitopes (4). These include antigens from the plant kingdom, insect venoms, and some components from nematode parasites (4-7). Since 2008, sensitization to another category of IgE-binding glycans including galactose-alpha-1,3-galactose (alpha-gal) which can be induced by bites of different tick species has been recognized (8-10). These oligosaccharides are best thought of as haptens because they can be expressed on proteins from multiple different species. Furthermore, in the case of alpha-gal the epitope for IgE ab can also be expressed on a lipid backbone (11, 12). There are many problems associated with including these epitopes in the allergen database, the most obvious of which is that none of them are related to a particular species, while the standard allergen nomenclature system is based on individual proteins, each produced by a species. In addition, the biosynthesis of an oligosaccharide is dependent on a series of enzymes related to each step in the process. Thus although the loss or inactivation of an enzyme can alter the structure of a glycan epitope, there is no such thing as a single gene that controls the production of an oligosaccharide (13, 14). Despite the obvious difficulties with incorporating glycan epitopes into the database, many members of the WHO/IUIS Allergen Nomenclature Sub-Committee had realized that the lack of a section on glycan epitopes in the database was increasingly irrational. There isn’t a simple way to classify the oligosaccharides since the IgE ab responses are not restricted to one group. In addition, in many cases it is difficult to define the species that induced a response. However, the first approach was to describe the general characteristics of the glycans related to pollens, venoms, nematode worms and ticks (2-4, 15). This approach identified ~20 oligosaccharides where there was clear evidence of their significance for allergic disease and which should be the initial proposal for the database. At this point it is clear that although there are biochemical conventions for the description of an oligosaccharide, there are several types of abbreviations, many of which are already in use. In this proposal, each oligosaccharide will be presented as a stick diagram with the accepted system for the individual monosaccharide residues (Fig 1). It is essential in any publication or presentation about an oligosaccharide to describe or illustrate its structure before using an abbreviation.
Symptoms Related to IgE ab Responses to Oligosaccharide Epitopes
The early studies related to CCD provided considerable evidence that IgE ab responses to these epitopes did not contribute to allergy symptoms (1, 3). This was particularly clear in relation to CCD epitopes on pollen antigens (2). The data is less clear in relation to venom antigens (7), but it has not been established that IgE ab specific for oligosaccharides play a significant role in acute allergic reactions to venom (15). Equally there is no good evidence that the symptoms that occur with nematodes entering the skin are related to IgE ab specific for oligosaccharides. There is extensive evidence about the presence of IgE ab to oligosaccharides on schistosomes and schistosome egg antigens (SEA) however, there have been very few studies on the relevance of these IgE ab with respect to symptoms. Thus, it remains unclear whether the intense itching that can occur with schistosomes of the pathogenic species or with cercaria of duck schistosomes (swimmer’s itch) are caused by or contributed to by IgE ab to oligosaccharides. The situation in relation to the mammalian oligosaccharide alpha-gal is completely different because in this case there is extensive evidence that severe symptoms both immediate during cetuximab infusions and reactions occurring 3-6 hours after eating red meat are directly related to specific IgE ab (8, 16-19). The delay before the start of symptoms after eating red meat in patients with the alpha-gal syndrome may be explained by the digestion of glycolipids (11, 12). Furthermore the digestion of glycolipids over 3-6 hours which allows the formation of low density lipoproteins (LDL) or HDL could be highly relevant to understanding the potential chronic effects of eating red meat (20, 21).
Definition of Epitopes
The glycan targets for IgE ab are by definition non-human, this is with the possible exception of IgE to the B blood group antigen which is immunogenic in humans who have A or O blood groups. Glycoproteins are formed by posttranslational attachment or assembly of the oligosaccharide moiety on the new synthesized protein in a process that generally occurs in two phases. In the production of N-linked oligosaccharides, the core structure starts with two GlcNAc monosaccharides attached to the side chain of asparagine (N) followed by a trimannosyl structure (Fig 1). The initial core structure is created in the Rough Endoplasmic Reticulum (RER) and is linked to asparagine (N) residues that are in the configuration of either NXS or NXT where S is serine, T threonine and X any amino acid except proline. There are a few examples of IgE ab binding epitopes consisting of O-linked glycans (2). An example of IgE ab binding to O-linked glycans is the mugwort pollen allergen Art v 1 and homologous allergens (Fig 2).
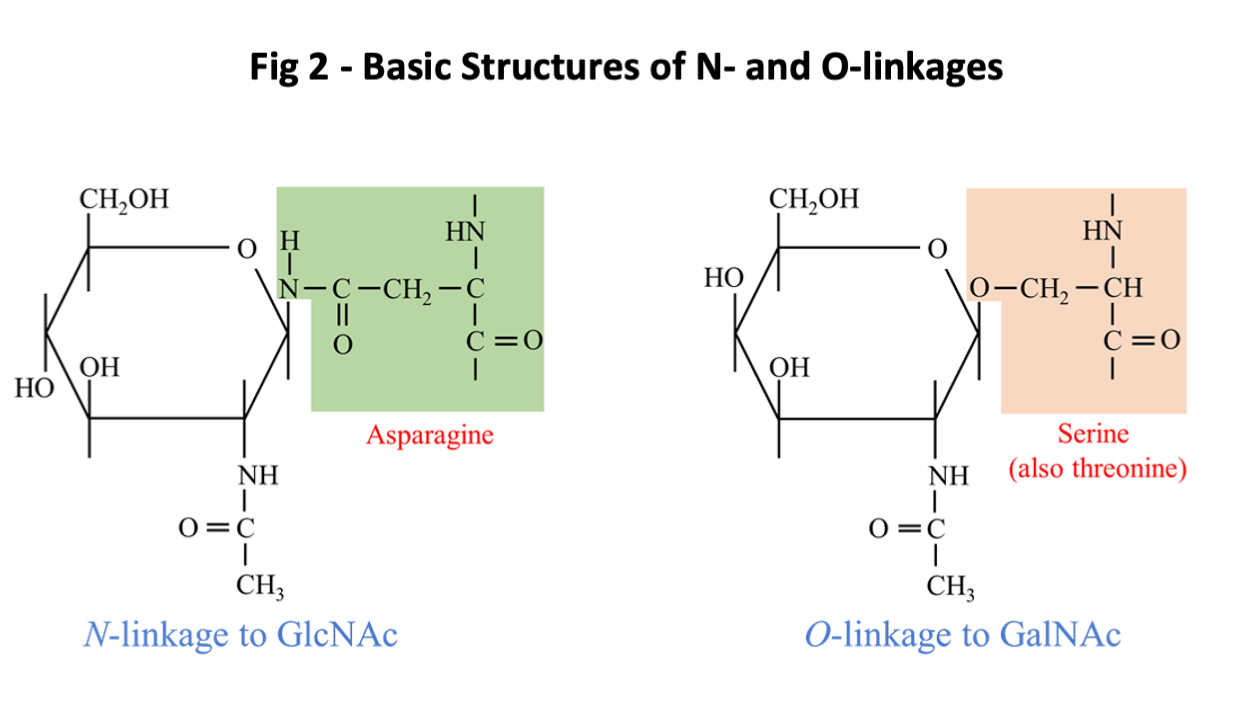
These N-linked and O-linked structures are clearly defined as parts of glycoproteins, and since the oligosaccharide can be attached to two commonly occurring sequence motifs, the structure is clearly not species specific (Fig 2). By contrast there are a few examples where a sugar structure contributes to a known protein allergen epitope, recognized by an antibody in a species-specific way. This situation has been clearly defined for an epitope on the cockroach allergen Bla g 2, where the role of a glycan as part of the epitope has been demonstrated in relation to both the binding of a monoclonal antibody and basophil histamine release (22, 23). In addition, the primary epitope for IgE ab binding on tomato allergen Sola l 2 (previously named Lyc e 2) has been shown to be MUXF (24). Similar data may be relevant to vespid venom allergen Ves m 2. Recent studies have also suggested that this “epitope-hapten” effect can also occur for alpha-gal (25). Following formation of the core glycan structure, saccharides may be trimmed or added in the Golgi apparatus in a process that requires several different enzymes. An oligosaccharide can be foreign to humans either because it includes a linkage that doesn’t occur in humans or because the structure includes non-human monosaccharides such as Xylose or the sialic acid, N-glycolyl neuraminic acid [Neu5Gc] (Fig 1). A specific feature for Neu5Gc as a non-human carbohydrate is that it can be absorbed from food and is readily incorporated into human glycoproteins (4). The antigenic and IgE-inducing properties of Neu5Gc are a matter of debate (4).
The descriptions in this section of the database will conform to the conventions established by the Consortium for Functional Glycomics (26). The oligosaccharides will be described in relation to four groups:
- Group A: Classical CCD’s (N-glycans) and O-glycans including oligosaccharides related to plants and venomous insects (Figs 1, 2, and 3).
- Group B: The nematode parasites and in particular the oligosaccharides that are present on schistosomes and schistosome Egg Antigens (SEA) (Fig 4).
- Group C: Mammalian non-human oligosaccharides including galactose alpha-1,3-galactose, and other monosaccharides such as N-glycolyl neuraminic acid. Alpha-gal is present in all mammals except great apes and Old World monkeys, but the route of sensitization in the USA, Australia, Europe and Japan is in almost all cases from one or more species of tick (Fig 5) (9, 10, 27-29).
- Group D: Short chain galacto-oligosaccharides (scGOS) produced from milk by bacterial beta-galactosidase (Fig 5)
Description of the four Groups:
Group A. Cross Reactive Carbohydrate Determinants: Classical CCD (N-glycans) and O-glycans
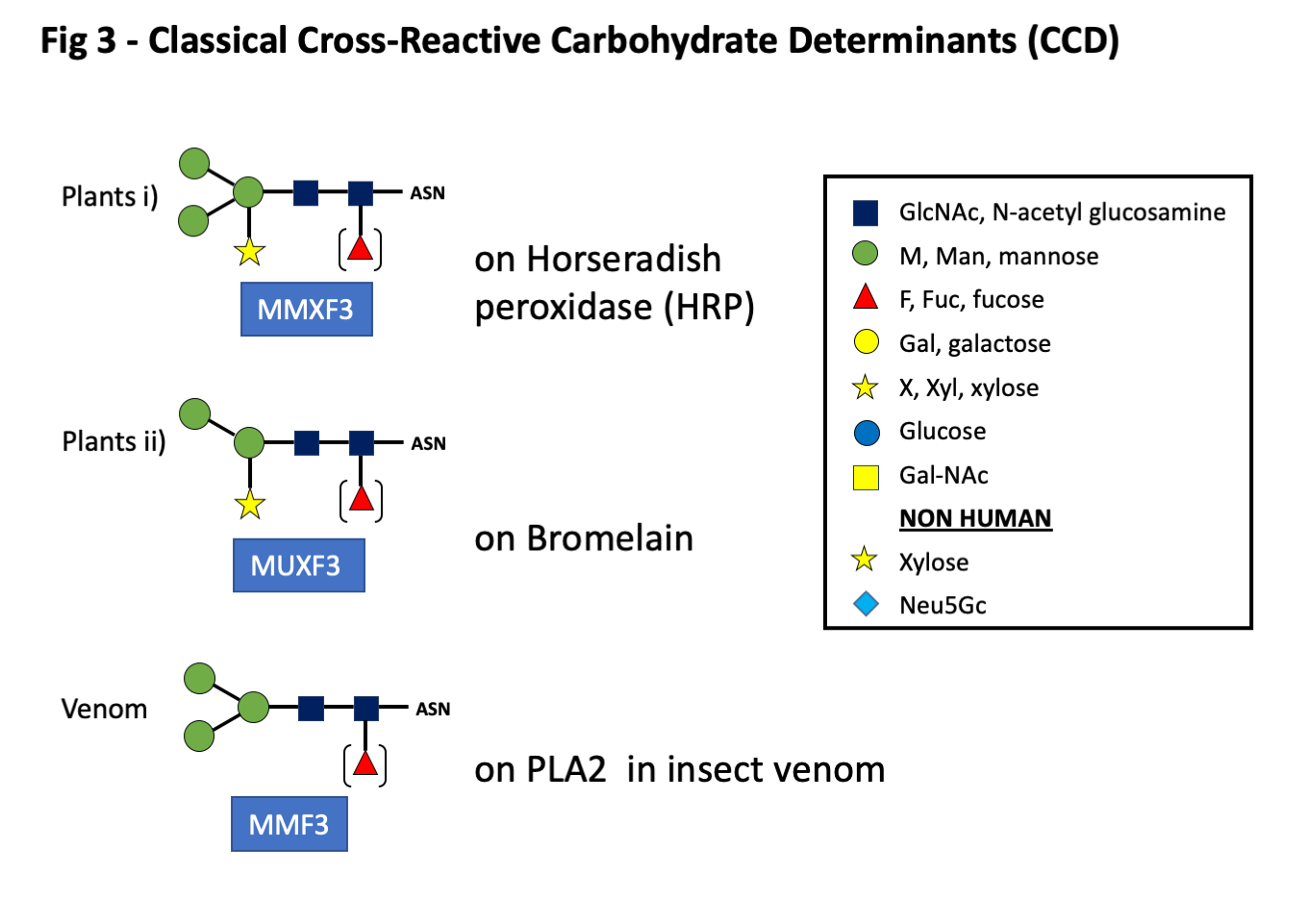
The first groups described in any detail were those on plant and venom proteins. These have a basic structure or core of two GlcNAc sugars with two or three terminal mannose residues. To these are added a xylose on the proximal mannose and/or a fucose residue linked alpha 1-3 on the first GlcNAc in the core (Fig 3). While these sugars were first recognized on plant proteins, e.g. bromelain from pineapple, similar oligosaccharides are present on proteins found in honeybee or wasp venom (Fig 3). Despite occasional cases where clinically significant reactions have occurred related to IgE ab to CCD, most investigators have concluded that antibodies to these sugars play little role in symptoms related to natural exposure (1-3). O-glycans, also included in this group, are carbohydrates attached to the side chain oxygen atom of serine or threonine residues in a protein. O-linked glycans are known to be present on ragweed Amb a 4, mugwort Art v1, grass pollen Phl p 1, and gum arabic from acacia, as well as some yeasts and molds.
Group B. Oligosaccharide Epitopes that are the Targets for IgE ab Responses to Schistosomes and other Helminths
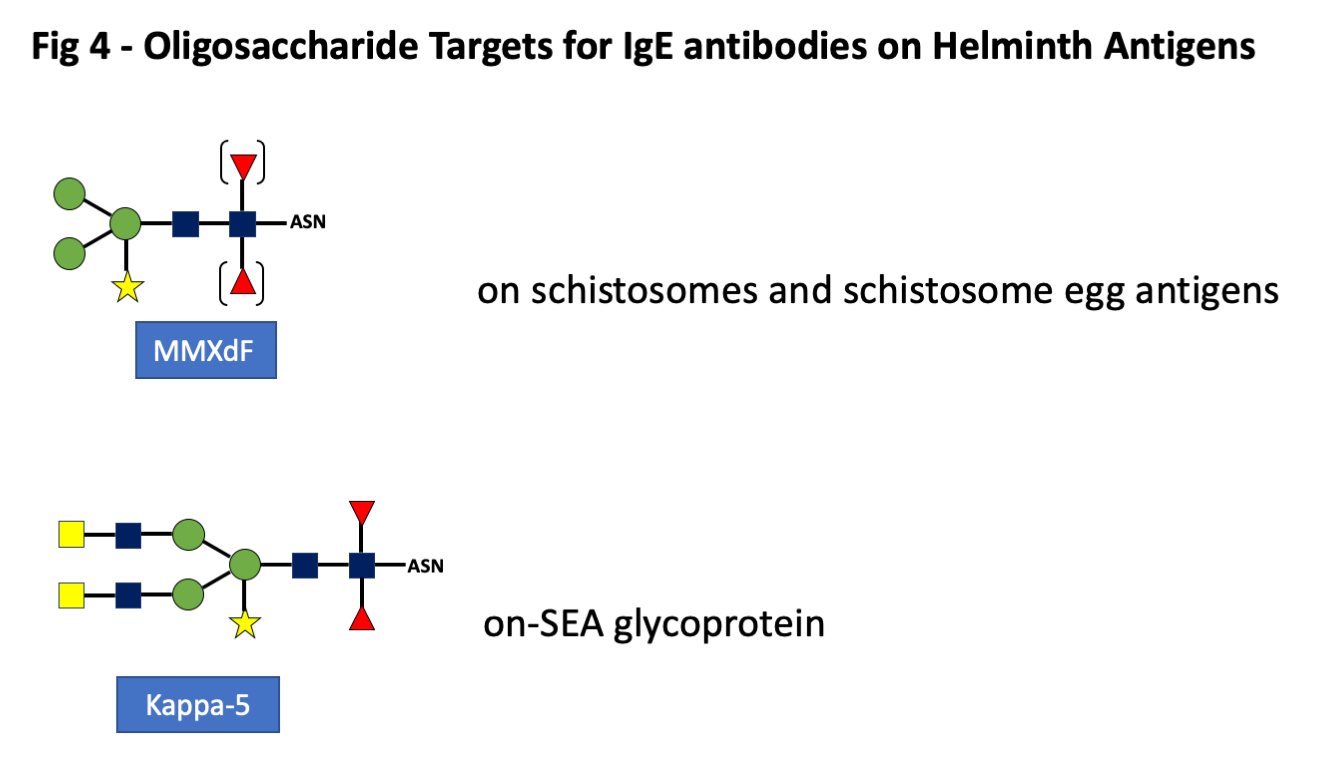
The existence of serum antibodies related to schistosomes or schistosome cercaria has been known since the earliest days of the Prausnitz-Kuestner test (30). The evidence that these antibodies against schistosome antigens were IgE was an important aspect of studies related to the early gatekeeper hypothesis of the role of IgE ab (31). The fact that these antibodies include specific IgE ab directed at oligosaccharides came from several investigators. In many cases these oligosaccharides are distinguished by a single terminal galactose or N-acetyl galactosamine residue (GalNAc) (Fig 4). Many different structures are found on other helminths, most of which are clearly foreign to humans but have not been fully investigated as targets of human IgE ab (4). Although the presence of oligosaccharides on other helminths is well established, there is relatively little evidence about the significance of these epitopes in relation to human IgE ab.
Group C. Mammalian non-human oligosaccharides including galactose-alpha-1,3-galactose, and other monosaccharides such as N-glycolyl neuraminic acid.
The third major group of glycan epitopes was first recognized by Karl Landsteiner who reported in 1925 that all humans had “natural” antibodies to a “B like” antigen that was present on the red blood cells of all non-primate mammals (32). This antigen was subsequently recognized as a major transplantation antigen of the non-primate mammals (32, 33). Dr. Uri Galili and others have done extensive work on the transplantation aspects of this antigen and they defined the structure as galactose-alpha-1,3-galactose (alpha-gal) (33). This oligosaccharide is indeed very close in structure to the blood group B antigens (Fig 5). In common with other blood group antigens, alpha-gal can be expressed on proteins or lipids (Fig 5) (11, 12). Thus, due to similarities with the B-antigen, individuals with blood group B have a reduced risk of sensitization to alpha-Gal in some but not all studies and a reduced risk of mammalian meat allergy (34, 35). IgE ab to alpha-gal were first recognized during the investigation of anaphylactic reactions to the monoclonal antibody cetuximab used in cancer treatment (8). This antibody is specific for Epidermal Growth Factor Receptor (EGFR) and is produced commercially in a cell line (SP2O) derived from mice (8). Those experiments demonstrated that the target of the IgE ab binding to cetuximab was an oligosaccharide which in most cases was the diantennary form of alpha-gal and was the dominant glycan epitope expressed on the variable region of the Fab portion of the heavy chain of the molecule (8). Furthermore, it was clear that these IgE ab were present in sera prior to the reaction, which occurred during the first infusion of cetuximab. Subsequent experiments demonstrated that in some areas of Virginia, North Carolina, Tennessee, and Arkansas, these IgE ab to alpha-gal were present in 15-20% of the adult population. It has also become clear that these IgE ab relate to tick bites in the USA and also in Australia, Sweden, France, Germany, Japan and many other countries (9, 10, 28, 29, 36-39). If patients with IgE ab to alpha-gal eat meat or organs from non-primate mammals, a proportion of them (between 5 and 20%) will experience an allergic or anaphylactic reaction which in most cases starts between 3 and 6 hours after eating meat (19, 40-42). The oligosaccharide target definitely includes the two terminal galactose sugars but may also be influenced by the adjacent monosaccharide which is GlcNAc (Fig 5). IgE ab can certainly bind to trisaccharide structures (43, 44). It is well established that the oligosaccharide, alpha-gal, is present on mammalian tissues either as part of a glycoprotein or a glycolipid. At present, it seems likely that the primary forms contributing to sensitization are glycoproteins. However, there are good reasons for thinking that the glycolipid forms of alpha-gal could at least contribute to the delay in symptoms after eating red meat (11, 19). What is certain is that the IgE ab response to this epitope can represent 40% or more of the total IgE in the circulation and that not surprisingly an IgE ab response to alpha-gal can lead to a rapid increase in total IgE (35). Sensitization to alpha-gal has also been found to be a risk factor for coronary artery disease (21). Finally, other non-human mammalian monosaccharides such as N-glycolyl neuraminic acid (Neu5Gc) can be included in this group (Figs 1 and 5) (44).
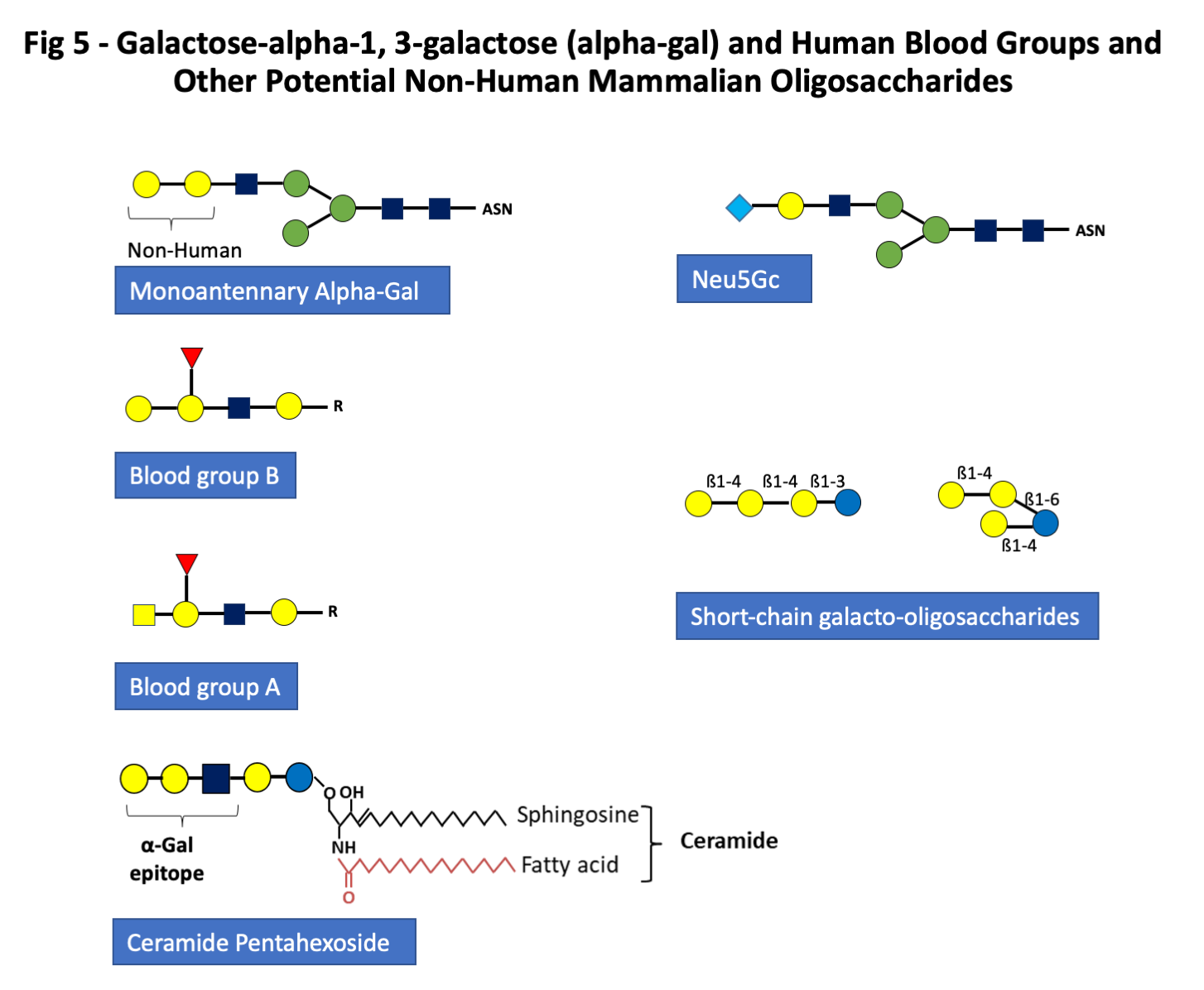
Group D: Short chain galacto-oligosaccharides (scGOS) produced by bacterial beta-galactosidase
GOS are commercially produced prebiotics consisting of mixtures of oligomers containing glucose and polymerized galactose units including ß1-6, ß1-4, and ß1-3 bonds (45). Allergic reactions to GOS have been reported mainly in Southeast Asia. Short chain GOS (scGOS) are produced through the enzymatic conversion of lactose, using beta-galactosidase derived from bacteria that are added to the commercial products (e.g. such as supplemented cow’s milk formula for infants). But they also occur naturally in human and animal milk. They typically consist of a chain of 2–6 galactose molecules attached to glucose (Fig 5). Contrary to the carbohydrates of the other 3 groups, scGOS are not bound to proteins in milk. Initially, occupational asthma in Japanese oyster farm workers was found to be associated with a carbohydrate linked to a high molecular weight allergen of sea squirt (46). A series of workers with sea squirt allergy showed immediate-type hypersensitivity reactions on ingestion of a lactic acid beverage commonly available in Japan. GOS present in that beverage gave positive results in skin and histamine release tests. The observed cross-reactivity between GOS and the sea squirt antigen suggested that the sea squirt was the sensitizing allergen source. When GOS was introduced in several milk formulas, the first cases of cow’s milk tolerant children who developed anaphylaxis following ingestion of cow’s milk formula supplemented with GOS were reported (47). Patients reacted to fractions of scGOS containing 3 sugar units or greater in skin prick test and basophil activation. However, not all scGOS are allergenic. Kaneko et al. were able to identify two allergenic scGOS and to develop a hypoallergenic one by using a beta-galactosidase from a different microbial source (48). Despite recent evidence from Singapore suggesting that sensitization to scGOS is related to dust mite sensitization, it remains true that prebiotics are widely available all over the world whereas allergic reactions seem to be limited to Asia, pointing to a still unknown primary sensitizing source (49).
References:
- 1. Aalberse RC, Koshte V, Clemens JGJ. Immunoglobulin-E Antibodies That Crossreact with Vegetable Foods, Pollen, and Hymenoptera Venom. J Allergy Clin Immunol. 1981;68(5):356-64.
- 2. Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142(2):99-115.
- 3. van Ree R. Clinical importance of cross-reactivity in food allergy. Curr Opin Allergy Clin Immunol. 2004;4(3):235-40.
- 4. Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: Sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol. 2017;140(2):356-68.
- 5. Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130(1):155-61.
- 6. van Diepen A, Smit CH, van Egmond L, Kabatereine NB, Pinot de Moira A, Dunne DW, Hokke CH. Differential anti-glycan antibody responses in Schistosoma mansoni-infected children and adults studied by shotgun glycan microarray. PLoS Negl Trop Dis. 2012;6(11):e1922.
- 7. Foetisch K, Westphal S, Lauer I, Retzek M, Altmann F, Kolarich D, Scheurer S, Vieths S. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111(4):889-96.
- 8. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109-17.
- 9. van Nunen SA. Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Med J Aust. 2018;208(7):316-21.
- 10. Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286-93 e6.
- 11. Wilson JM, Platts-Mills TA. The oligosaccharide galactose-α-1, 3-galactose and the α-Gal syndrome: insights from an epitope that is causal in immunoglobulin E-mediated immediate and delayed anaphylaxis. EMJ Allergy Immunol. 2018;3:89-98.
- 12. Roman-Carrasco P, Lieder B, Somoza V, Ponce M, Szepfalusi Z, Martin D, Hemmer W, Swoboda I. Only alpha-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy. 2019;74(10):1956-68.
- 13. Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, Goodman M. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci U S A. 2007;104(2):559-64.
- 14. Galili U. Evolution in primates by "Catastrophic-selection" interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am J Phys Anthropol. 2019;168(2):352-63.
- 15. Jappe U, Raulf-Heimsoth M, Hoffmann M, Burow G, Hubsch-Muller C, Enk A. In vitro hymenoptera venom allergy diagnosis: improved by screening for cross-reactive carbohydrate determinants and reciprocal inhibition. Allergy. 2006;61(10):1220-9.
- 16. O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644-8.
- 17. Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol. 2015;135(3):589-96; quiz 97.
- 18. Hilger C, Hemmer W, Swoboda I, Morisset M, Fischer J, Tripathi A, Platts-Mills T, Biedermann T. Molecular and extract-based diagnostics in meat allergy. Molecular Allergy Diagnostics: Springer; 2017. p. 305-26.
- 19. Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, Mozzicato S, Platts-Mills TA. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134(1):108-15.
- 20. Mosedale DE, Chauhan A, Schofield PM, Grainger DJ. A pattern of anti-carbohydrate antibody responses present in patients with advanced atherosclerosis. J Immunol Methods. 2006;309(1-2):182-91.
- 21. Wilson JM, Nguyen AT, Schuyler AJ, Commins SP, Taylor AM, Platts-Mills TAE, McNamara CA. IgE to the Mammalian Oligosaccharide Galactose-alpha-1,3-Galactose Is Associated With Increased Atheroma Volume and Plaques With Unstable Characteristics-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38(7):1665-9.
- 22. Li M, Gustchina A, Glesner J, Wunschmann S, Vailes LD, Chapman MD, Pomes A, Wlodawer A. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. J Immunol. 2011;186(1):333-40.
- 23. Do DC, Yang S, Yao X, Hamilton RG, Schroeder JT, Gao P. N-glycan in cockroach allergen regulates human basophil function. Immun Inflamm Dis. 2017;5(4):386-99.
- 24. Westphal S, Kolarich D, Foetisch K, Lauer I, Altmann F, Conti A, Crespo JF, Rodriguez J, Enrique E, Vieths S, Scheurer S. Molecular characterization and allergenic activity of Lyc e 2 (beta-fructofuranosidase), a glycosylated allergen of tomato. Eur J Biochem. 2003;270(6):1327-37.
- 25. Jappe U, Minge S, Kreft B, Ludwig A, Przybilla B, Walker A, Varga R, Seidel P, Biedermann T, Anemuller W, Kromminga A, Rueff F, Merk H, Wagner N, Treudler R, Worm M, Waldmann I, Saloga J, Becker WM, Goldmann T, Platts-Mills TA, Homann A. Meat allergy associated with galactosyl-alpha-(1,3)-galactose (alpha-Gal)-Closing diagnostic gaps by anti-alpha-Gal IgE immune profiling. Allergy. 2018;73(1):93-105.
- 26. Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, Prestegard JJ, Schnaar RL, Freeze HH, Marth JD, Bertozzi CR, Etzler ME, Frank M, Vliegenthart JF, Lutteke T, Perez S, Bolton E, Rudd P, Paulson J, Kanehisa M, Toukach P, Aoki-Kinoshita KF, Dell A, Narimatsu H, York W, Taniguchi N, Kornfeld S. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology. 2015;25(12):1323-4.
- 27. Apostolovic D, Mihailovic J, Commins SP, Wijnveld M, Kazimirova M, Starkhammar M, Stockinger H, Platts-Mills TAE, Cirkovic Velickovic T, Hamsten C, van Hage M. Allergenomics of the tick Ixodes ricinus reveals important alpha-Gal-carrying IgE-binding proteins in red meat allergy. Allergy. 2020;75(1):217-20.
- 28. Hashizume H, Fujiyama T, Umayahara T, Kageyama R, Walls AF, Satoh T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-α-1, 3-galactose carbohydrate IgE antibody levels: a retrospective cohort study in a single institution. J Am Acad Dermatol. 2018;78(6):1135-41. e3.
- 29. Chinuki Y, Morita E. Alpha-Gal-containing biologics and anaphylaxis. Allergol Int. 2019;68(3):296-300.
- 30. Taliaferro WH, Taliaferro LG. Skin reactions in persons infected with Schistosoma mansoni. Puerto Rico J Publ Hlth. 1931;7:23-35.
- 31. Steinberg P, Ishizaka K, Norman PS. Possible Role of IgE-Mediated Reaction in Immunity. J Allergy Clin Immunol. 1974;54(6):359-66.
- 32. Landsteiner K. The Specificity of Serological Reactions, 1936. Baltimore, MD. 1936.
- 33. Galili U. Significance of the evolutionary alpha1,3-galactosyltransferase (GGTA1) gene inactivation in preventing extinction of apes and old world monkeys. J Mol Evol. 2015;80(1):1-9.
- 34. Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of α‐Gal‐and B‐antigen‐specific responses differentiates red meat‐allergic patients from healthy individuals. Allergy. 2018;73(7):1525-31.
- 35. Wilson JM, Schuyler AJ, Workman L, Gupta M, James HR, Posthumus J, McGowan EC, Commins SP, Platts-Mills TAE. Investigation into the alpha-Gal Syndrome: Characteristics of 261 Children and Adults Reporting Red Meat Allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348-58 e4.
- 36. Hamsten C, Starkhammar M, Tran T, Johansson M, Bengtsson U, Ahlén G, Sällberg M, Grönlund H, van Hage M. Identification of galactose‐α‐1, 3‐galactose in the gastrointestinal tract of the tick I xodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68(4):549-52.
- 37. Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29(8):841-9.
- 38. Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, James H, van Berkel PH, van de Winkel JG, Platts-Mills TA, Parren PW. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29(7):574-6.
- 39. Wilson JM, Keshavarz B, Retterer MKC, Workman L, Schuyler A, McGowan E, Lane C, Kandeel A, Purser J, Ronmark E, LaRussa J, Commins S, Merritt T, Platt-Mills TAE. A dynamic relationship between two regional causes of IgE-mediated anaphylaxis: α-Gal syndrome and imported fire ant. J Allergy Clin Immunol. 2020;In Press.
- 40. Levin M, Apostolovic D, Biedermann T, Commins SP, Iweala OI, Platts-Mills TAE, Savi E, van Hage M, Wilson JM. Galactose alpha-1,3-galactose phenotypes: Lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598-602.
- 41. Hilger C, Fischer J, Wolbing F, Biedermann T. Role and Mechanism of Galactose-Alpha-1,3-Galactose in the Elicitation of Delayed Anaphylactic Reactions to Red Meat. Curr Allergy Asthma Rep. 2019;19(1):3.
- 42. Kiewiet MBG, Apostolovic D, Starkhammar M, Grundstrom J, Hamsten C, van Hage M. Clinical and Serological Characterization of the alpha-Gal Syndrome-Importance of Atopy for Symptom Severity in a European Cohort. J Allergy Clin Immunol Pract. 2020.
- 43. Apostolovic D, Krstic M, Mihailovic J, Starkhammar M, Cirkovic Velickovic T, Hamsten C, van Hage M. Peptidomics of an in vitro digested alpha-Gal carrying protein revealed IgE-reactive peptides. Sci Rep. 2017;7(1):5201.
- 44. Apostolovic D, Tran TAT, Sánchez‐Vidaurre S, Cirkovic Velickovic T, Starkhammar M, Hamsten C, van Hage M. Red meat allergic patients have a selective IgE response to the α‐Gal glycan. Allergy. 2015;70(11):1497-500.
- 45. Soh JY, Huang CH, Lee BW. Carbohydrates as food allergens. Asia Pacific allergy. 2015;5(1):17-24.
- 46. Ohta M, Shigeta S, Ono K, Takao T, Shimonishi Y, Oka S. Sugar sequences of allergenically active oligosaccharide alcohols isolated from a large-molecular-size sea squirt antigen termed H-antigen. Arch Biochem Biophys. 1989;275(1):151-65.
- 47. Chiang WC, Huang CH, Llanora GV, Gerez I, Goh SH, Shek LP, Nauta AJ, Van Doorn WA, Bindels J, Ulfman LH, Knipping K, Delsing DJ, Knol EF, Lee BW. Anaphylaxis to cow's milk formula containing short-chain galacto-oligosaccharide. J Allergy Clin Immunol. 2012;130(6):1361-7.
- 48. Kaneko K, Watanabe Y, Kimura K, Matsumoto K, Mizobuchi T, Onoue M. Development of hypoallergenic galacto-oligosaccharides on the basis of allergen analysis. Biosci Biotechnol Biochem. 2014;78(1):100-8.
- 49. Lee LYGN, Zhong Y, Leow SY, Lim SC, Wen H, Soh JY, Chiang WC, Delsing DJ, Lee BW, Huang C-H. Allergy to prebiotic galacto-oligosaccharides: House dust mites—the putative primary sensitizer. J Allergy Clin Immunol. 2020;145(2):707-10. e5.
Should you have additional information about carbohydrate epitopes or suggestions for improvement of the current document, please let us know [contact Dr. Anna Pomés: apomes (at) inbio.com].